Short Answer
Calculate E for the titration of 50.00 mL of 0.100 M Ag+ when 50.00 mL of 0.150 M Cl− is added.Silver wire is the indicator electrode and the saturated Ag-AgCl electrode is the reference electrode.E = 0.197 V for the saturated Ag-AgCl electrode, 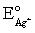 = 0.7993 V and Ksp = 1.8 × 10−8 for AgCl.
= 0.7993 V and Ksp = 1.8 × 10−8 for AgCl.
Definitions:
Related Questions
Q1: A pH probe is calibrated against pH
Q5: The pH of a weak-acid solution is
Q8: Which statement is TRUE for the leveling
Q14: Discuss the focus of the voice of
Q16: A student must prepare 500.0 mL of
Q16: Calculate the percentage dissociation for a 0.010
Q16: The concentration and absorbance data below was
Q19: Which equation is NOT required to determine
Q68: With respect to the six competencies summarized
Q276: One way that governments can improve market