A student is given the assignment to determine the mass iron in a brand of women's vitamins.He begins by taking four vitamin tablets,grinding the tablets into a fine powder to undergo the necessary sample preparation to free all iron from the sample matrix and to reduce all iron to the +2 state.The resulting Fe2+ solution is transferred to a 250 mL volumetric flask and diluted to volume with distilled water.A 50 mL aliquot of the sample requires 42.98 mL of 1.00 x 10−3 M 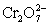 .Calculate the mg Fe per tablet.
.Calculate the mg Fe per tablet. 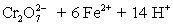 ⇋
⇋ 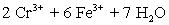
Definitions:
Cost of Goods Sold
Costs incurred directly from producing the goods a company offers, encompassing labor and material expenses.
Physical Inventory
A physical count of merchandise or commodities an organization has on hand at a specific time.
FOB Shipping Point
A term used in shipping contracts to indicate that the buyer assumes responsibility for the goods and the shipping costs from the point of departure.
FOB Shipping Point
A term used in shipping agreements that means "Free on Board Shipping Point," indicating that the buyer is responsible for freight and other shipping costs from the seller's location.
Q2: Small organizations and nonprofits have generally been
Q2: _ is used to isolate a single
Q7: Calculate the volume of an aquarium that
Q21: The government has just passed a law
Q41: The term "invisible hand" was coined by<br>A)
Q123: The term "market failure"<br>A) means the same
Q142: When you calculate your true costs of
Q210: Causes of market failure include<br>A) externalities and
Q266: Senator Smart,who understands economic principles,is trying to
Q272: The average cost per seat on the