1.25 g C6H5COOH is dissolved in water and the solution is titrated with 33.5 mL of a solution of NaOH of an unknown concentration.Calculate the normality of the NaOH for the reaction C6H5COOH(aq) + NaOH(aq) → C6H5COONa(aq) + H2O( 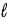 ) .
) .
Definitions:
Whistle-blowing
The act of exposing any kind of information or activity that is deemed illegal, unethical, or not correct within an organization by an insider.
Social Responsibility Continuum
A range that depicts the varying degrees to which businesses may engage in acts intended to benefit society, from minimal to extensive involvement.
State Uncertainty
Refers to the inability to predict future states of the environment, making decision-making challenging due to unknown variables.
Environmental Forces
External factors such as social, economic, technological, competitive, and regulatory conditions that affect an organization's operations and outcomes.
Q12: A sample of solid silver releases 476
Q19: Which of the following correctly applies to
Q20: The hydroxide ion concentration of a solution
Q22: Which of the following statements is/are correct?<br>i.Some
Q24: What is the oxidizing agent in this
Q28: Consider the following thermochemical equation: CaO(s)+ H<sub>2</sub>O(
Q42: Which of the following compounds is/are (an)amide(s)?<br>i.CH<sub>3</sub>-NH<sub>2</sub><br>ii.(CH<sub>3</sub>)<sub>2</sub>-NH<br>iii.(CH<sub>3</sub>)<sub>3</sub>-N<br>iv.CH<sub>3</sub>-CO-NH<sub>2</sub><br>v.CH<sub>3</sub>(CH<sub>2</sub>)<sub>2</sub>-CO-NH<sub>2</sub><br>A)i
Q43: Which of the following has a linear
Q49: In Heinrich Müller: Big-Four Whistleblower,Müller had an
Q68: The term "true and fair view" tends