ΔH = +572 kJ for the decomposition of water by the reaction 2 H2O( 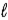 ) → 2 H2(g) + O2(g) .How many grams of water can be decomposed by 5.00 × 103 kJ of energy?
) → 2 H2(g) + O2(g) .How many grams of water can be decomposed by 5.00 × 103 kJ of energy?
Definitions:
Great Expectations
A novel by Charles Dickens, often used metaphorically to represent scenarios where there are high hopes and anticipations.
Obscure Facts
Information that is not widely known or is hidden from common knowledge.
Inverted Pyramid
The traditional structure of news writing and the writing of news releases.
Organizational Goals
Objectives set by a business or organization to guide operations and decision-making.
Q5: Tommy Hubbs is the new controller of
Q21: DeGeorge thinks that "corporations have a moral
Q23: Which of the following is NOT a
Q23: Which of the following is the best
Q29: The statement about leadership attributed to John
Q31: The "particularity" provision in the PSLRA allows
Q35: The International Federation of Accountants (IFAC) research
Q53: Kelly and Jordan are writing a term
Q60: Which of the following is NOT a
Q74: Organizational ethics can be thought of as:<br>A)Descriptions