ΔH = +572 kJ for the decomposition of water by the reaction 2 H2O( 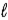 ) → 2 H2(g) + O2(g) .How many grams of water can be decomposed by 5.00 × 103 kJ of energy?
) → 2 H2(g) + O2(g) .How many grams of water can be decomposed by 5.00 × 103 kJ of energy?
Definitions:
Behavioral Learning
A theory that explains how people learn new behaviors through conditioning, either by associating two stimuli or by rewards and punishments.
Cue
A signal or stimulus that triggers a specific response or action, often used in behavior and perception.
Drive
An internal state that motivates an individual to satisfy needs or achieve goals.
Response
In the feedback loop, the impact the message had on the receiver’s knowledge, attitudes, or behaviors during the communication process.
Q8: Lotus Hospitality,a U.S.publicly-owned company doing business in
Q12: What is the pH of a solution
Q19: The Private Securities Litigation Reform Act imposes
Q22: What is the molecular geometry surrounding the
Q23: Which of the following substances is/are homogeneous?<br>i.Mineral
Q29: The thermochemical equation describing the heat change
Q39: Most methods for separating mixtures into their
Q43: The hydrogen ion concentration of a solution
Q53: The difference between errors in the financial
Q92: Which of the following is NOT something