Use the standard half-cell potentials listed below to calculate the standard cell potential for the following reaction occurring in an electrochemical cell at 25°C.(The equation is balanced. )
3 Cl2(g) + 2 Fe(s) → 6 Cl⁻(aq) + 2 Fe3+(aq) 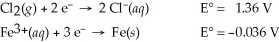
Definitions:
Assessment Interview
A structured conversation used by practitioners to evaluate the condition, needs, or performance of an individual, often utilized in psychological and medical contexts.
Active Intervention
Proactive measures taken to address or resolve problems, typically in a clinical or therapeutic context, aiming for positive change.
Assessment Interview
A structured conversation between a healthcare provider and a patient aimed at gathering comprehensive information to evaluate the patient's health status or psychological condition.
Sudden Change
An abrupt alteration or transformation in a situation, behavior, or condition that occurs unexpectedly.
Q12: Calculate the pOH of a solution that
Q13: Which metal can be oxidized with a
Q26: Calculate ΔG<sub>rxn</sub> at 298 K under the
Q42: Which of the following situations is impossible?<br>A)An
Q50: Identify oxidation.<br>A)increase in oxidation number<br>B)loss of electrons<br>C)gain
Q53: Q = 1<br>A)ΔG < 0<br>B)standard state<br>C)ΔG >
Q59: What is the oxidizing agent in the
Q62: Three forces, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg" alt="Three forces,
Q66: Consider two vectors <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg" alt="Consider two
Q82: Use the standard half-cell potentials listed below