Use the tabulated half-cell potentials below to calculate the equilibrium constant (K) for the following balanced redox reaction at 25°C.
2 Al(s) + 3 Mg2+(aq) → 2 Al3+(aq) + 3 Mg(s) 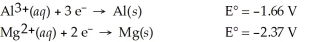
Definitions:
Accounts Receivable Subsidiary Ledger
A detailed ledger that tracks individual accounts receivable transactions and balances for customers.
Cash Receipts Journal
An accounting journal specifically used to record all receipts of cash, detailing the source of each receipt for a given period.
Record Payment
The process of documenting the payment of a bill or an obligation in the business’s financial records.
Revenue Journal
The revenue journal is a ledger book or digital record in accounting that tracks all incoming earnings or revenue transactions generated by business operations.
Q3: What is the pH of a 0.020
Q8: Consider the following reaction,equilibrium concentrations,and equilibrium constant
Q31: Identify the symptom that is not from
Q44: Determine the pOH of a 0.227 M
Q48: An object is moving with constant non-zero
Q66: Use the tabulated half-cell potentials below to
Q69: The graph in the figure shows the
Q74: Which of the following numbers is the
Q113: If the acceleration of an object is
Q147: A 1.0 L buffer solution is 0.050