Use the standard half-cell potentials listed below to calculate the standard cell potential for the following reaction occurring in an electrochemical cell at 25 °C.(The equation is balanced. )
2 K(s) + I2(s) → 2 K⁺(aq) + 2 I⁻(aq) 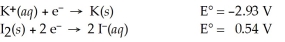
Definitions:
Nonrecurring Gains
Profits that are not expected to happen regularly or repeatedly, coming from events like asset sales, lawsuit winnings, or one-time events affecting financial performance.
Income From Continuing Operations
Earnings generated from the normal, recurring activities of a business, excluding any one-time transactions or discontinued operations.
Nonrecurring Losses
Losses that are not expected to happen again in the foreseeable future, differentiated from normal business operations.
Accounting Events
Transactions or occurrences that result in changes to the financial statements of a company, requiring recognition or disclosure.
Q9: Place the following in order of decreasing
Q15: Give the expression for the solubility product
Q28: Use the standard half-cell potentials listed below
Q37: Consider the following reaction: Xe(g)+ 2 F<sub>2</sub>(g)→
Q43: Give the pH of 0.330 M phosphoric
Q44: Three vectors, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6214/.jpg" alt="Three vectors,
Q46: What is (0.674/0.74). Expressed to the correct
Q55: Determine the pH of a 0.741 M
Q79: A reasonable estimate for the height of
Q95: A jet plane is launched from a