Calculate the cell potential for the following reaction that takes place in an electrochemical cell at 25°C.
Cu(s) ∣ Cu2+(aq,0.0032 M) ∣∣ Cu2+(aq,4.48 M) ∣ Cu(s) 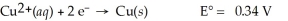
Definitions:
Estimated
Refers to approximate calculations or judgments about figures or values that are not precisely known.
Finished Goods Inventory
Inventory that consists of items that have completed the manufacturing process but have not yet been sold to customers.
Budgeted
Pertaining to amounts or figures that have been planned for in a budget, predicting future income and expenditures.
Manufacturing Overhead
All indirect costs associated with manufacturing, excluding direct materials and direct labor costs.
Q1: Why aren't solids or liquids included in
Q2: Calculate the pH of a 1.60 M
Q18: Calculate the hydronium ion concentration in an
Q26: Arrhenius acid<br>A)electron pair donor<br>B)proton donor<br>C)produces protons in
Q28: Use the standard half-cell potentials listed below
Q49: An object moves 15.0 m north and
Q52: Suppose that a car traveling to the
Q55: In which of the following processes do
Q63: Estimate how many pennies would you have
Q86: A 100.0 mL sample of 0.10 M