At what temperature would the root mean square speed of oxygen molecules,O2,be 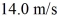
If oxygen behaves like an ideal gas? The mass of one O2 molecule is 5.312 × 10-26 kg,and the Boltzmann constant is 1.38 × 10-23 J/K.
Definitions:
Centration
A cognitive limitation in early child development, where the focus is on one salient aspect of a situation, neglecting other possible factors, as described in Piaget's theory of cognitive development.
Egocentrism
A cognitive characteristic where an individual has difficulty understanding or acknowledging a perspective other than their own.
Narcissism
A personality trait characterized by an exaggerated sense of self-importance, need for admiration, and lack of empathy for others.
Disparity
A great difference or inequality between two or more things, often leading to an unfair situation.
Q2: A 0.50-kg object is attached to an
Q8: A 5-µF,a 7-µF,and an unknown capacitor C<sub>X</sub>
Q14: At what temperature would the root mean
Q16: A container with rigid walls is filled
Q17: A ball is attached to an ideal
Q32: A pendulum that was originally erected by
Q33: A pump uses a piston of 15
Q35: A plastic rod is charged up by
Q38: A 90-g aluminum calorimeter contains 390 g
Q122: When a 1.0-m length of metal wire