An expansion process on an ideal diatomic ideal gas for which CV = 5/2 R has a linear path between the initial and final coordinates on a pV diagram.The coordinates of the initial state are: the pressure is 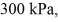
The volume is 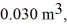
And the temperature is 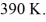
The final pressure is 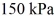
And the final temperature is 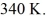
What is the change in the internal (thermal) energy of the gas,during this process? (R = 8.31 J/mol ∙ K)
Definitions:
Spontaneous Recovery
The reappearance of a conditioned response after a period of lessened response.
Operant Conditioning
A technique for adjusting behavior by dispensing rewards for good actions or penalties for bad ones.
Delayed Conditioning
A classical conditioning process where the neutral stimulus is presented and followed by the unconditioned stimulus after a brief interval.
Monthly Reward
A regular incentive or benefit given once a month, often as recognition for achievements or maintained standards.
Q11: An incompressible ideal fluid flows steadily through
Q19: If 50 g of lead (of specific
Q37: What is the resistance of 1.0 m
Q44: The density of material at the center
Q49: A proton is placed in an electric
Q72: An ideal gas is held in a
Q73: The force of attraction that a -40.0
Q73: The weather outside is frightful.The temperature is
Q104: A 4.0-Ω resistor is connected to a
Q207: A multiloop circuit is shown in the