Multiple Choice
Given the table of specific heat values below,what is the identity of a 26.2 g metal sample that increases by 8.5°C when 100.0 J of energy is absorbed? 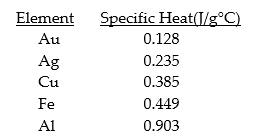
Definitions:
Related Questions
Q4: Which state of matter has atomic spacing
Q11: How many Calories are in 575.0 calories?<br>A)575,000<br>B)0.5750<br>C)137.6<br>D)2,404<br>E)none
Q15: A spectator ion is one that does
Q18: The mole has a value of 6.023
Q23: The heat capacity of a substance is
Q55: What is the base SI unit for
Q57: If two atoms each contain different numbers
Q58: Chemicals make up everything around you,including your
Q74: Consumer-generated online marketing efforts to promote brands
Q185: Which tool would organizations want to use