Multiple Choice
How many kJ of heat are needed to completely vaporize 3.30 moles of H2O?
The heat of vaporization for water at the boiling point is 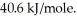
Definitions:
Related Questions
Q22: Balance the redox reaction in acid solution:
Q23: What is the concentration of the hydronium
Q51: The volume of a gas and the
Q57: In the reaction S + O<sub>2 </sub>→
Q65: Which of the following atoms has the
Q84: The fact that the oceans contain salt
Q90: A gas may not behave ideally under
Q102: Water has a heat of vaporization of
Q111: Calculate the molarity of a KCl solution
Q112: A reaction that has K<sub>eq </sub>= 2.0