Multiple Choice
A 250 gram sample of water at the boiling point had 45.0 kJ of heat added.How many grams of water were vaporized?
Heat of vaporization for water is 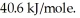
Definitions:
Related Questions
Q11: What is the balanced oxidation half-reaction for
Q20: Consider the three compounds below,then choose the
Q22: Chlorine has 8 valence electrons in the
Q28: Sodium hydroxide is often used in the
Q48: A 90.0 g sample of NaOH is
Q56: The volume of a gas is independent
Q71: Reaction rates generally increase as a reaction
Q109: If a slow leak in an inner
Q110: For the reaction KMnO<sub>4</sub> + Li →
Q111: For the reaction 2 H<sub>2</sub>O (l)⇌ 2H<sub>2</sub>