How many moles of H2O are formed from the complete combustion of 45.0 g of methanol, CH3OH? 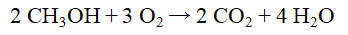
Definitions:
Potentially Useful
Describes information, tools, or processes that may be beneficial or advantageous in certain contexts or under specific conditions.
Innovative Ideas
Refers to novel or original concepts, methods, or products that can potentially bring about significant improvements or changes.
Sponsorship And Support
Refers to the provision of financial, material, or emotional assistance to individuals or organizations, often to promote specific events, activities, or causes.
Managerial Practices
The methods, strategies, and techniques employed by managers to achieve organizational objectives and manage employee performance.
Q14: In the reaction given below, how many
Q20: The molar mass of calcium hydroxide, Ca(OH)<sub>2</sub>
Q21: Which bond is shortest?<br>A) carbon-oxygen double bond<br>B)
Q29: Ions that contain atoms of more than
Q34: Five coins are tossed. Which combination of
Q36: Starting with a saturated solution of sodium
Q40: Samples of the following isotopes in pure
Q46: Brittle behavior in rocks leads to formation
Q47: Arrange the solutions in order of increasing
Q49: At constant T and P, in which