In the reaction given below, how many grams of sodium metal are consumed if 2.02 g of hydrogen gas are produced? 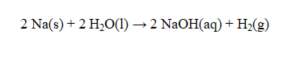
Definitions:
Supply Curve
A graphical representation of the relationship between the price of a good and the quantity supplied.
Loanable Funds
The money available in the banking system for lending to businesses or consumers, influenced by interest rates and monetary policy.
Demand for Loanable Funds
The Demand for Loanable Funds represents the relationship between the interest rate and the total amount of loans that borrowers are willing to take at that rate.
Technological Advance
The introduction of new technologies or the improvement of existing ones, enhancing productivity and efficiency.
Q4: The Roman numerals in the reaction given
Q4: The Hawaiian Islands were formed by repeated
Q8: Which statement about strong acids is true?<br>A)
Q13: Which substance has the highest molar heat
Q14: Use the VSEPR model to predict the
Q26: Give the element that has the electron
Q39: Caffeine has the empirical formula C<sub>4</sub>H<sub>5</sub>N<sub>2</sub>O and
Q40: An electrolytic reaction is a system
Q53: In the reaction shown, the radiation produced
Q55: Assume all hydrocarbons given are linear. Which