If 110.0 g of iron reacts with 64.0 g of oxygen, what is the theoretical yield of Fe2O3? 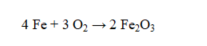
Definitions:
Effective Rate
The actual interest rate that borrowers pay or investors receive on a financial product, once all the compounding periods are factored in, often higher than the nominal rate.
Compounding Interval
The frequency at which interest is applied to the principal sum of a loan or deposit, affecting the total interest earned or paid.
Compounded Nominal Rate
The rate of interest quoted for a period, usually a year, without taking into account the effect of compounding within that period.
Effective Rate
The actual interest rate of an investment or loan, taking into account the effect of compounding interest as opposed to the nominal or stated rate.
Q6: Discuss what role Rutherford's gold foil experiment
Q7: If solid sodium chloride is added to
Q21: Use the VSEPR model to predict the
Q22: The most common minerals in the crust
Q31: Which interaction(s) exist(s) between CO molecules? <img
Q33: Rocks that are formed by the crystallization
Q35: The rule that the mass number of
Q43: All of these are common modes of
Q48: According to the First Law of Thermodynamics,
Q49: How many electrons can the second principal