Consider the reaction 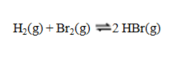 If the partial pressures in an equilibrium mixture of H2, Br2, and HBr are 0.024 atm, 0.031 atm, and 5.07 atm, respectively, the value of Kp for this reaction at this temperature is
If the partial pressures in an equilibrium mixture of H2, Br2, and HBr are 0.024 atm, 0.031 atm, and 5.07 atm, respectively, the value of Kp for this reaction at this temperature is
Definitions:
Buyer's Place
A term referring to the designated location where goods are to be delivered or where a transaction is completed in a sales contract.
Destination Contract
A contract in which the seller is required to ship the goods by carrier and deliver them at a particular destination. The seller assumes liability for any losses or damage to the goods until they are tendered at the destination specified in the contract.
Risk of Loss
The potential for an investment or venture to result in financial loss, often evaluated as part of investment strategies and decision-making.
Carrier
A company or individual that undertakes the professional conveyance of goods or people.
Q30: Suppose that 1.00 gram of each of
Q38: Consider the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="Consider the
Q38: Consider the reaction<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5061/.jpg" alt="
Q39: How many significant figures are present in
Q42: According to band theory, a good conductor
Q47: What is the proper answer and number
Q53: Which of the following is a metal?<br>A)
Q54: An aqueous solution of phosphoric acid, H<sub>3</sub>PO<sub>4</sub>,
Q54: A particular reaction mixture (K<sub>p</sub> = 10)
Q57: Which of the following best describes a