Consider the reaction 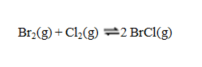 If the partial pressures in an equilibrium mixture of Br2, Cl2, and BrCl are 1.12 atm, 1.26 atm, and 3.14 atm, respectively, the value of Kp for this reaction at this temperature is
If the partial pressures in an equilibrium mixture of Br2, Cl2, and BrCl are 1.12 atm, 1.26 atm, and 3.14 atm, respectively, the value of Kp for this reaction at this temperature is
Definitions:
Self-Reported Health
An individual's assessment or perception of their own health status, often used in health surveys and research.
Optimists
Individuals who generally believe that positive events are more likely to occur than negative ones in the future.
Good Events
Occurrences that have a favorable outcome or bring happiness, well-being, or satisfaction.
Longitudinal Evidence
Data gathered from the same subjects repeatedly over a period of time to observe changes and developments, providing insight into effects and patterns that emerge over the long term.
Q6: Recovering aluminum directly from its ore, which
Q6: The activation energy in the Arrhenius equation
Q20: In a chemical reaction, 36 g of
Q22: If 50. mL of solution containing 2.0
Q29: Prior to the implementation of the Affordable
Q37: What are the main activities lobbyists engage
Q40: An electrolytic reaction is a system
Q43: Which correctly describes the relationship between the
Q49: Which pair of substances represents a conjugate
Q54: Which has the lowest entropy at a