Multiple Choice
The decomposition of N2O(g) to nitrogen and oxygen has the rate law 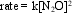 where k = 1.1 × 10-3 M-1 s-1 at 565 C. If the initial concentration of a sample of N2O is 0.50 M, what is the concentration after one hour at 565 C?
where k = 1.1 × 10-3 M-1 s-1 at 565 C. If the initial concentration of a sample of N2O is 0.50 M, what is the concentration after one hour at 565 C?
Comprehending the role of asset allocation and selection within markets in contributing to total excess return.
Learning how the geometric average rate of return is calculated and its basis.
Recognizing the importance of observation periods and variance in evaluating portfolio performance.
Identifying the creators and the concept behind the M2 measure.
Definitions:
Related Questions
Q1: In what ways is regulatory policy in
Q5: When the external pressure on a balloon
Q15: Which of the following categories of taxes
Q23: All of the following contribute to legislative
Q31: The following is the reaction that occurs
Q35: Which statement is false?<br>A) Amorphous solids have
Q37: Exhibit 11-1 For the following question(s), consider
Q39: How many significant figures are present in
Q52: How many of the following processes
Q56: Which condition represents the most acidic solution?<br>A)