The decomposition of N2O(g) to nitrogen and oxygen has the rate law 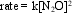 where k = 1.1 × 10-3 M-1 s-1 at 565 C. If the initial concentration of a sample of N2O is 0.50 M, what is the concentration after one hour at 565 C?
where k = 1.1 × 10-3 M-1 s-1 at 565 C. If the initial concentration of a sample of N2O is 0.50 M, what is the concentration after one hour at 565 C?
Definitions:
Aptitude
A natural ability or talent for learning or skill in a particular area.
Cultural Psychologists
Researchers who study how cultural practices shape psychological processes and vice versa.
Customs
Traditional practices or behaviors shared by a particular culture or society.
Traditions
Customs or beliefs passed down from generation to generation, often forming a part of a community's cultural or social identity.
Q5: When the external pressure on a balloon
Q6: What are collective benefits? Why do governments
Q8: Which unit is appropriate for describing
Q8: List the four minor parties of the
Q9: Which of the following taxes CANNOT be
Q10: Which set of conditions describes a reaction
Q14: _ containing compounds are known as organic
Q37: Compare and contrast benefit-based taxes and taxation
Q38: Although aluminum is a moderately reactive metal,
Q46: According to band theory, a semiconductor is