Determine the volume (in L) of Cl2(g) required to carry out the following reaction at 794 torr and 625°C using 15.0 g of Fe. The value of R = 0.0821 L atm mol-1 K-1. 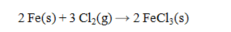
Definitions:
Virtual Organization
A business model that operates online, often without a traditional physical presence, relying heavily on information and communication technologies.
Temporary Venture
A project or enterprise undertaken for a limited duration, often to achieve a specific goal or address a particular need.
Special Expertise
A specific skill set or knowledge that differentiates an individual or organization in a particular area.
Systems/Contingency
A theory or approach that emphasizes the need to adapt management strategies or organizational structures based on the current environment and specific situations.
Q12: Explain why city residents pay twice for
Q14: _ refers to the items that are
Q16: The Texas Veterans Leadership Program,established in 2008
Q18: What office was created under the 1836
Q22: Calculate the time needed to plate out
Q23: How are school districts financed in Texas?
Q32: Consider an electrochemical cell as shown, with
Q45: The least common form of local government
Q64: An enzyme is a(n)<br>A) organic or inorganic
Q67: Which of the following is not a