At 25°C, the following heats of reaction are known: 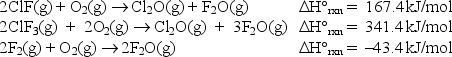 At the same temperature, use Hess's law to calculate H°rxn for the reaction: ClF(g) + F2(g) ClF3(g)
At the same temperature, use Hess's law to calculate H°rxn for the reaction: ClF(g) + F2(g) ClF3(g)
Definitions:
Greenmail
A situation where a company buys back its own shares from a potential acquirer at a price higher than the market value to avoid a takeover.
Poison Pill
A poison pill is a defense strategy used by a corporation to deter or prevent hostile takeovers.
Takeover Attempt
An effort by one company or entity to gain control of another company by acquiring a significant portion of its shares or assets.
Defensive Merger Tactics
Strategies employed by a company to avoid being taken over by another company, often including legal and financial maneuvers.
Q21: Calculate the molar mass of a gaseous
Q32: How many grams of N<sub>2</sub>O, nitrous oxide,
Q32: Thorium metal is prepared by reacting
Q33: Which one of the following does not
Q38: Complete this statement: Coulomb's law states that
Q40: Given 2Al(s)+ (3/2)O<sub>2</sub>(g) <span class="ql-formula" data-value="\rarr"><span
Q54: Use the Born-Haber cycle to calculate
Q83: A gas is compressed in a cylinder
Q91: Give five examples of elements that occur
Q108: Aluminum hydroxide reacts with nitric acid to