Calculate the Energy Change for the Reaction K(g)+ Br(g) K+(g)+ Br- (G)
Given the Following Ionization Energy (IE)and Electron
Calculate the energy change for the reaction K(g) + Br(g) K+(g) + Br- (g)
Given the following ionization energy (IE) and electron affinity (EA) values 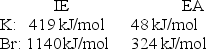
Definitions:
Tourist Expenditures
Amount of money spent by visitors in a country on accommodation, food, transportation, entertainment, and other services and goods.
Unilateral Transfers
Financial flows from one country to another that do not require repayment, such as foreign aid or remittances.
Balance of Payments
A record of all economic transactions between the residents of a country and the rest of the world within a certain period.
Exports
Products or services transferred from one nation to another for the purpose of sale or exchange.
Q10: The molar enthalpy of vaporization of carbon
Q14: Which of the following make an isoelectronic
Q46: A 26.2 g piece of copper metal
Q46: What is the charge on the monatomic
Q68: The residential rate for natural gas is
Q71: Write the ground-state electron configuration for Mg<sup>2+</sup>.
Q96: Find the heat absorbed from the
Q100: Indicate the type of hybrid orbitals used
Q105: How many electrons in a ground-state cadmium
Q118: A piece of zinc metal was added