Assume that the conductivity of a solution depends only on the total concentration of dissolved ions and that you measure the conductivity of three different solutions while performing titrations in which
I.50.00 mL of 0.100 M aqueous CH3CO2H is titrated by addition of 0.100 M NaOH.
II.50.00 mL of 0.100 M aqueous NaBr is titrated by addition of 0.100 M AgNO3.
III.50.00 mL of 0.100 M aqueous CaCl2 is titrated by addition of 0.100 M Na2CO3. 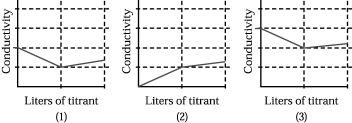
-Which of the above graphs corresponds to titration III?
Definitions:
Problem-solving
The process of identifying an issue and developing, implementing, and assessing solutions to rectify or improve the situation.
Decision-making
The process of making choices by identifying a decision, gathering information, and assessing alternative resolutions.
Diversity
The inclusion or representation of different types of people (in terms of race, age, sex, ethnicity, sexual orientation, etc.) in a group or organization.
Selection Decision
The process by which an employer evaluates and decides upon which candidates will be offered a job from a pool of applicants.
Q10: Disproportionation is a reaction in which a
Q37: The compound K<sub>2</sub>S is predicted to be
Q87: Which of the following is true?<br>A)The Bohr
Q122: Molecular orbitals extending over more than two
Q131: Consider a molecule with the following connections:
Q157: Which are isotopes? An atom that has
Q167: Which pair of reactants will produce a
Q172: Calculate the energy change for the formation
Q184: The paramagnetism of O<sub>2</sub> is explained by<br>A)coordinate
Q199: The fundamental SI unit for measuring matter