Which atomic orbitals are involved in bonding and which as lone pair orbitals for N2H2? 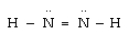
Definitions:
Cupcakes
Small, individually-sized cakes that are typically frosted and decorated, often used to celebrate events or milestones.
Total Surplus
Total surplus is the sum of consumer surplus and producer surplus in a market, representing the total benefits to both buyers and sellers from trade.
Producer Surplus
The difference between the amount that producers are willing to sell a good for and the actual amount received by them.
Producer Surplus
The difference between the amount producers are willing to accept for a good or service and the actual amount they receive.
Q2: Which is the most exothermic reaction?<br>A)CH<sub>4</sub>(g)+ 2
Q88: What is the ground-state electron configuration of
Q100: Of the following,which has the shortest de
Q115: What are the possible values of n
Q115: What is the ground-state electron configuration of
Q122: Which picture corresponds to potassium fluoride?<br>A)picture (a)<br>B)picture
Q132: Effusion of a 1:1 mixture of two
Q135: An electron in a 4p orbital can
Q144: Calculate the energy change in kJ/mol for
Q158: When the equation MnO<sub>4</sub><sup>-</sup>(aq)+ C<sub>2</sub>O<sub>4</sub><sup>2-</sup>(aq)→ Mn<sup>2+</sup>(aq)+ CO<sub>2</sub>(g)is