Effusion of a 1:1 mixture of two gases,represented by unshaded and shaded spheres in the diagram below,through a small pinhole produces the result shown below.The shaded spheres have a molecular mass of 20 amu.Which gas molecules have the higher average speed and what is the molecular mass of the unshaded molecules? 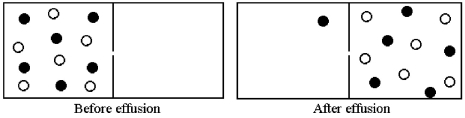
Definitions:
Experience
The knowledge or skill acquired by a period of practical involvement in an activity or exposure to events or people over time.
Auditory Senses
The sensory system related to hearing, allowing individuals to perceive sounds through the process of detecting vibrations.
Upbeat Music
Music with a fast tempo and a positive, cheerful mood, often used to uplift and energize listeners.
External Social Environment
Factors outside an organization that affect its operation, including social trends, cultural norms, and demographic shifts.
Q2: Which is the most exothermic reaction?<br>A)CH<sub>4</sub>(g)+ 2
Q22: The reaction shown below has the rate
Q41: At constant pressure,the combustion of 5.00 g
Q58: The electronegativity is 2.1 for H and
Q61: Which thermodynamic function is most related to
Q76: Acetylene torches utilize the following reaction:<br>2 C<sub>2</sub>H<sub>2</sub>(g)+
Q84: Which of the following pairs of solutions
Q107: How many electrons are in the valence
Q176: A solution is prepared by dissolving 40.0
Q183: Which ion-dipole interaction results in the larger