Calculate the enthalpy of combustion per mole for C6H12O6.Assume that the combustion products are CO2(g) and H2O(l) . 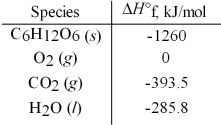
Definitions:
Santa Maria
A term that can refer to various churches, ships, and places, often associated with Christian contexts or explorations, notably the flagship of Christopher Columbus on his first voyage to the New World.
Andrea Pisano
An Italian sculptor and architect, active in the 14th century, renowned for his work on the Baptistery of San Giovanni in Florence and his contributions to Gothic art.
Christian Virtues
Moral standards and qualities valued in Christianity, such as faith, hope, and charity, guiding ethical behavior and spiritual wellbeing.
Andrea Pisano
An Italian sculptor and architect from the 14th century, known for his works in Florence, including the campanile of the Florence Cathedral.
Q20: What is the temperature of CO<sub>2</sub> gas
Q23: Which of the following is not equivalent
Q33: Isoelectronic means having the same number of
Q54: What are the bond angles in the
Q71: Kinetic energy increases with increasing _ and
Q108: A spontaneous reaction has a _ value
Q121: Calculate the energy change for the formation
Q136: The following four spheres represent a Na
Q213: What is the hybridization of the carbon
Q230: What is the hybridization of the carbon