A phase diagram of temperature versus composition for a mixture of the two volatile liquids octane (bp = 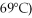 and decane (bp = 126°C) is shown.
and decane (bp = 126°C) is shown. 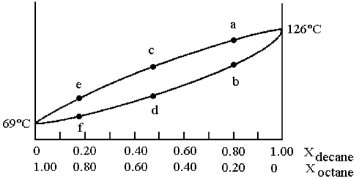
-Assume that you start with a mixture containing 0.80 mol of decane and 0.20 mol of octane,what is the vapor composition at the boiling point?
Definitions:
Traits
Enduring characteristics or qualities that define an individual's personality.
Early Childhood
A developmental stage that covers the period from birth to 8 years of age.
Fully Functioning Person
A term used in humanistic psychology to describe an individual who is living in alignment with their true self, experiencing an ongoing process of self-actualization and personal growth.
Unconditional Positive Regard
An attitude of acceptance and respect on the part of an observer, no matter what a person says or does.
Q20: If K<sub>c</sub> equals 0.11 at 25°C for
Q30: The coolant in automobiles is often a
Q46: What is the empirical formula of the
Q51: According to Le Châtelier's principle,if the volume
Q56: Which of the following statements are true
Q61: The number of moles of ions in
Q115: Aqueous solutions of 30% (by weight)hydrogen peroxide,H<sub>2</sub>O<sub>2</sub>,are
Q117: What is the pressure in a gas
Q127: For the process: HNO<sub>3</sub>(g)⇌ HNO<sub>3</sub>(l)<br>ΔH° is -39.04
Q157: Cyclohexane (C<sub>6</sub>H<sub>12</sub>)undergoes a molecular rearrangement in the