Multiple Choice
Shown below is a concentration vs.time plot for the reaction A ⇌ 2B.For this reaction the value of the equilibrium constant is 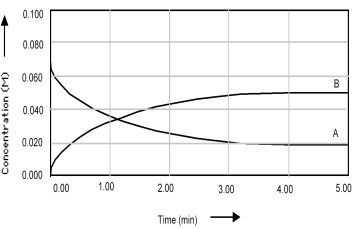
Calculate projected dividend amounts under various corporate policies and market conditions.
Understand how stock dividends and repurchases influence a firm's financial statements and market valuation.
Interpret the effects of changes in dividend policies on shareholder value and company capital structure.
Analyze the financial outcomes of specific corporate actions, including stock splits and dividend changes.
Definitions:
Related Questions
Q21: What is the equilibrium equation for the
Q35: If solution (1)is a saturated solution of
Q71: What is the pH at the equivalence
Q75: Which solution has the largest percent dissociation
Q98: Identify the Br∅nsted-Lowry bases.<br>A)(1)and (3)<br>B)(1)and (4)<br>C)(2)and (3)<br>D)(2)and
Q112: What is the entropy of 10<sup>5</sup> molecules
Q123: Predict the sign of ΔS for each
Q169: What is the pH of a 0.40
Q177: At 55° the decomposition of N<sub>2</sub>O<sub>5</sub> is
Q179: Potassium chromate is slowly added to a