Picture (1) represents an equilibrium mixture of solid CaCO3,solid CaO,and gaseous CO2,obtained as a result of the endothermic decomposition of CaCO3. 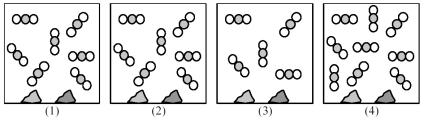
-Which picture (2) -(4) represents the equilibrium mixture after addition of four more CO2 molecules?
Definitions:
Net Working Capital
The difference in a company’s readily available assets versus its current financial obligations, marking its short-term economic status.
Statement of Financial Positions
A financial report that presents a company's assets, liabilities, and equity at a specific point in time, giving insight into its financial health.
Comprehensive Income
The total change in equity for a reporting period other than from transactions with owners, including all revenues, gains, expenses, and losses.
Q27: A solution with a hydroxide ion concentration
Q98: What is the pH of a solution
Q121: Determine the acid dissociation constant for a
Q121: In aqueous solution,hypobromite ion,BrO<sup>-</sup>,reacts to produce bromate
Q134: Which nonequilibrium mixture will react in the
Q141: The following pictures represent mixtures of cis-C<sub>2</sub>H<sub>2</sub>X<sub>2</sub>
Q180: An acidic solution at 25°C will have
Q182: What is the pH of a 0.020
Q189: What is the weight percent of vitamin
Q191: Two aqueous solutions,A and B,are separated by