What is the molar solubility of AgCl in 0.10 M NaCN if the colorless complex ion Ag(CN) 2- forms? Ksp for AgCl is 1.8 × 10-10 and Kf for Ag(CN) 2- is 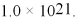
Definitions:
Layout Tab
An interface element within software applications where users can adjust page or document layout and formatting options.
Vertical Ruler
A tool displayed alongside a document or workspace, providing a vertical scale for layout measurements and alignment.
Update the Bibliography
The process of revising a list of references or works cited in a document to include new sources or correct information.
Cursive
A style of handwriting where the letters are written in a flowing manner, often for the purpose of faster writing.
Q3: What percentage of a radioactive substance remains
Q18: What is the balanced equation for the
Q26: The enthalpy for the following reaction is
Q62: What is the approximate pH of a
Q77: A reaction for which ΔH° = +
Q81: Indicate the coefficient in front of H<sub>2</sub>O<sub>2</sub>
Q100: Which points a-d represent the half-equivalence point
Q144: For the isomerization reaction:<br>Butane ⇌ isobutane<br>K<sub>p</sub> equals
Q144: Which is a net ionic equation for
Q198: The percent dissociation of acetic acid changes