The following plot shows two titration curves,each representing the titration of 50.00 mL of 0.100 M acid with 0.100 M NaOH. 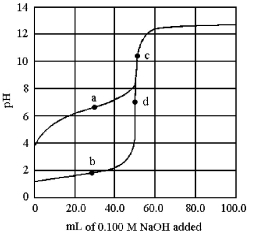
-Which points a-d represent the half-equivalence point and the equivalence point,respectively,for the titration of a weak acid?
Definitions:
Professional Boundaries
The ethical and legal limits that define the relationships between professionals and their clients or patients.
Critical Thinking
The practice of considering all aspects of a situation when deciding what to believe or what to do.
Stress Management
Techniques and strategies used to control stress levels and improve one’s ability to cope with challenges.
Communication Skills
the ability to convey or share ideas and feelings effectively through verbal and non-verbal methods.
Q20: If K<sub>c</sub> equals 0.11 at 25°C for
Q22: If the concentrations of Ag<sup>+</sup>(aq)and Cu<sup>2+</sup>(aq)are varied
Q58: In which set are all compounds considered
Q60: For initial state 2 what is the
Q72: What is not a commercial method for
Q81: Which of the solutions are buffer solutions?<br>A)(1)and
Q89: The entropy of water at 25° is
Q142: State whether the solubility of Cu(OH)<sub>2</sub> will
Q166: For the reaction shown below,which change in
Q194: Identify the Br∅nsted-Lowry acid/base conjugate pairs.<br>A)(1)/(2)and (3)/(4)<br>B)(1)/(3)and