The following pictures represent solutions that contain a weak acid HA and/or its potassium salt KA.Unshaded spheres represent H atoms and shaded spheres represent A- ions.(K+,H3O+,OH-,and solvent H2O molecules have been omitted for clarity. ) 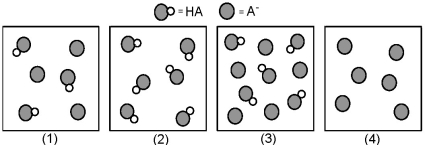
-Which of the solutions are buffer solutions?
Definitions:
Cornell University
A prestigious Ivy League research university located in Ithaca, New York, known for its diverse array of undergraduate and graduate programs.
Fee-Based Service
A type of service that requires payment of a specific fee in exchange for access to the service or assistance provided.
Court Opinions
Written statements by judges explaining their decisions in a particular case, often including legal reasoning and precedents.
Agency Materials
Documents and resources that are owned or used by an agency in the conduct of its duties.
Q25: Which of the above reaction mixtures has
Q37: In the relationship ΔG = -nFE°,what is
Q43: Without doing any calculations,determine whether the standard
Q82: What is the Henderson-Hasselbalch equation for the
Q113: Predict the sign of ΔS of the
Q121: Determine the acid dissociation constant for a
Q132: What is the entropy of 10 molecules
Q187: Undersea flora prefer a maximum concentration of
Q202: From the following chemical reactions determine the
Q221: Dihydrogen phosphate H<sub>2</sub>PO<sub>4</sub><sup>-</sup>,has an acid dissociation constant