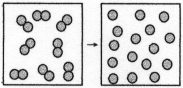
-The figure above represents the reaction O2(g) → 2O(g) ,which is nonspontaneous at 25°C.How will the spontaneity of this reaction vary with temperature? This reaction is
Definitions:
Portfolio Required Return
The minimum expected return on an investment portfolio that an investor is aiming for, based on their investment goals and risk tolerance.
Beta
A measure of a stock's volatility in relation to the overall market, indicating its relative risk.
Standard Deviation
A measure of the amount of variation or dispersion of a set of values; used in finance to quantify the risk associated with a security's return.
Market Risk
The risk of losses in investments attributable to market-wide phenomena, such as economic changes or interest rate fluctuations.
Q2: Which element shown on the periodic table
Q4: Which solution has the greatest buffer capacity?<br>A)(1)<br>B)(2)<br>C)(3)<br>D)(4)
Q19: In general,as a reaction goes to equilibrium<br>A)ΔG
Q37: What is an appropriate method for the
Q41: What is k in Boltzmann's formula,S =
Q55: What is the pH of a solution
Q69: At 2600 K,ΔG° = 775 kJ for
Q100: Arrange the acids in order of increasing
Q144: Identify the Lewis acid that acts as
Q151: If solution (1)is a saturated solution of