Consider the following gas-phase reaction of A2 (shaded spheres) and B2 (unshaded spheres) :
A2(g) + B2(g) ⇌ 2 AB(g) ΔG ° = +25 kJ 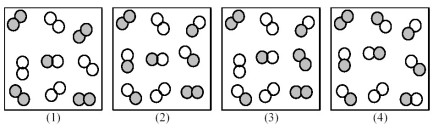
-Which of the above reaction mixtures has the most spontaneous forward reaction?
Definitions:
Francis Ford Coppola
An acclaimed American film director, producer, and screenwriter, known for directing the "Godfather" series and "Apocalypse Now," among other films.
Unique Aesthetic
A distinctive visual style or artistic approach that sets a work or artist apart from others.
Tim Burton
A director, producer, and artist known for his gothic fantasy and horror films featuring dark, quirky themes and distinctive characters.
Blockbuster Franchise
A series of movies that are commercially very successful, often featuring extensive merchandising and multiple sequels.
Q27: What is the approximate pH at the
Q41: What is the relationship between the standard
Q47: In which compound does oxygen have a
Q57: Which of the following reactions is most
Q61: At 25°C,?G°<sub>f </sub>is -620 kJ/mol for
Q78: Which compound contains hydrogen atoms that form
Q79: O<sub>2</sub>(g)+ 4 H<sup>+</sup>(aq)+ 4 e<sup>-</sup>→ 2 H<sub>2</sub>O(l)E°
Q103: Which pair of ions can be separated
Q136: Which of the above reaction mixtures is
Q156: What are the allotropes of oxygen?<br>A)O and