Multiple Choice
Consider the following galvanic cell. 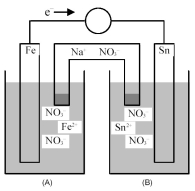
-Identify the anode and cathode,and indicate the direction of Na+ ion and NO3- ion flow from the salt bridge.
Definitions:
Related Questions
Q22: What is not a correct expression for
Q24: Calculate the standard free energy for the
Q42: At 25°C CO<sub>2</sub> is a gas and
Q93: What is the total volume of hydrogen
Q116: What is the pH of 1 L
Q133: What statement is most inconsistent about the
Q141: Which is most often used in the
Q166: What is the reaction of potassium peroxide
Q169: Which of the following can function as
Q169: What is the pH of the resulting