Given:
4AlCl3(s) + 3O2(g) → 2Al2O3(s) + 6Cl2(g) ; ΔH = -529.0 kJ
Determine ΔH for the following thermochemical equation.
Cl2(g) +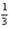 Al2O3(s) →
Al2O3(s) → 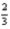 AlCl3(s) +
AlCl3(s) +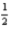 O2(g)
O2(g)
Definitions:
Line Chart
A graph that displays information as a series of data points called 'markers' connected by straight line segments, often used to visualize trends over time.
Frequencies
The number of times a particular data point appears in a set of data.
Nominal Variable
A variable used in statistics to label or categorize without a natural order or ranking.
Interval
A range of values between two points, often used in mathematics and statistics to denote a specific span of numbers.
Q7: Who postulated that energy is radiated only
Q17: When 20.0 g C<sub>2</sub>H<sub>6</sub> and 60.0 g
Q30: Which of the following atoms is paramagnetic
Q40: How many molecules are there in 1.54
Q48: Which of the following represents a known
Q67: Consider the following energy-level diagram for a
Q78: How many moles of gas are in
Q106: The formation of which monatomic ion of
Q111: For the reaction that occurs in a
Q141: The formula for aluminum sulfate is<br>A)Al<sub>3</sub>(SO<sub>4</sub>)<sub>2</sub>.<br>B)Al<sub>3</sub>S<sub>2</sub>.<br>C)Al<sub>2</sub>(SO<sub>4</sub>)<sub>3</sub>.<br>D)Al<sub>2</sub>S<sub>3</sub>.<br>E)Al<sub>2</sub>(SO<sub>3</sub>)<sub>3</sub>.