Which of the following statements is false concerning the reaction of hydrogen gas and oxygen gas given below?
H2(g) + 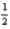
O2(g) → H2O(l) ; ΔH = -285.8 kJ
Definitions:
Ovulating
The part of the menstrual cycle where an ovary releases an egg, making it available for fertilization.
Casual Sex
Sexual activity that takes place outside the context of a serious relationship and without the expectation of commitment.
Cognitive Component
The aspect of attitude that consists of beliefs and knowledge about a subject.
Affective Component
Part of an attitude that involves an individual’s feelings or emotions about the attitude object.
Q3: Which substance has a standard enthalpy of
Q21: Given the following data,calculate the standard enthalpy
Q21: 2Al(s)+ 6HCl(aq)→ 2AlCl<sub>3</sub>(aq)+ 3H<sub>2</sub>(g)<br>According to the equation
Q47: Which of the following is a nonelectrolyte
Q66: The branch of physics that mathematically describes
Q69: Which of the following would not be
Q81: Analysis of a compound containing only C
Q90: Under conditions of constant pressure,for which of
Q95: The approximate CCO angle in acetone, <img
Q97: The molar mass of an unknown gas