Given the following data,calculate the standard enthalpy of reaction for the conversion of buckminsterfullerene (C60) into diamond: 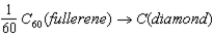 C(graphite) → C(diamond) ; ΔH° = +1.897 kJ
C(graphite) → C(diamond) ; ΔH° = +1.897 kJ
60C(graphite) → C60(fullerene) ; ΔH° = +2193 kJ
Definitions:
21st-Century
The current century, spanning from 2001 to 2100, characterized by rapid technological advancements, globalization, and significant social and environmental changes.
Road Series
A series of comedy films featuring Bob Hope, Bing Crosby, and Dorothy Lamour, known for their exotic settings and improvised style.
Hope and Crosby
Refers to Bob Hope and Bing Crosby, who partnered in a series of popular "Road to…" comedy films, combining humor and musical sequences.
Melodrama
A dramatic genre characterized by exaggerated plot and characters, intended to appeal to the emotions of the audience.
Q19: What is the maximum number of electrons
Q40: Which of the following has a standard
Q60: Given the molecular orbital diagram for dinitrogen
Q61: A π bond is the result of
Q62: Calculate the change in enthalpy when 52.0
Q62: How many valence electrons are present in
Q76: An impure sample of benzoic acid (C<sub>6</sub>H<sub>5</sub>COOH,122.12
Q93: In which of the following species is
Q100: How many molecules are there in 104
Q121: Which of the following species represents an