What mass of oxygen is consumed when 285.5 kJ of energy is evolved from the combustion of a mixture of H2(g) and O2(g) ?
H2(g) + 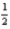
O2(g) → H2O(l) ; ΔH° = -285.8 kJ
Definitions:
Forged Check
A check that has been illegally signed or altered by someone other than the account owner.
Obligated
A condition or situation where one is legally or morally bound to perform a certain action or fulfill a duty.
Stop-Payment Order
a request made to a bank by an account holder not to pay a check that has been issued but not yet cashed.
Loss
A decrease in value, resources, or money typically as a result of a business transaction, investment, or unforeseen events.
Q11: Consider the following series of molecular ions
Q20: In the Lewis dot formula for the
Q55: Which molecule or ion has a trigonal
Q57: What is ΔH° for the following reaction?<br>2C<sub>2</sub>H<sub>2</sub>(g)+
Q60: Consider the following changes at constant temperature
Q67: What is the formula for the nitride
Q90: A compound is composed of only C
Q102: Which of the following species cannot function
Q113: Using bond-energy data,what is ΔH° for the
Q136: A sample of 96 g of ozone,O<sub>3</sub>,contains