What mass of oxygen is consumed when 285.5 kJ of energy is evolved from the combustion of a mixture of H2(g) and O2(g) ?
H2(g) + 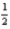
O2(g) → H2O(l) ; ΔH° = -285.8 kJ
Definitions:
REM Sleep
A stage of sleep characterized by rapid eye movements, paralysis of major muscles, faster breathing, and dreaming.
Animal Magnetism
An historical term referring to a supposed invisible natural force possessed by all living beings, proposed by Franz Mesmer.
Hypnotic Trances
Hypnotic trances are states of deep relaxation and heightened focus induced by hypnosis, often used to explore psychological issues, habits, or to produce changes in behavior.
Eccentrics
Individuals who deviate from the accepted norms in behavior, appearance, or manner, often seen as unconventional or odd.
Q29: In Bohr's atomic theory,when an electron moves
Q41: In basic solution the chromate ion,CrO<sub>4</sub><sup>2-</sup>,can be
Q55: Use the bond energies provided to complete
Q59: The partial pressures of CH<sub>4</sub>,N<sub>2</sub>,and O<sub>2</sub> in
Q62: Which of the following sets of four
Q63: The elements that are filling the 5f
Q86: What is the standard enthalpy change for
Q87: For the resonance hybrid of the nitrite
Q91: The molecule AX<sub>3</sub>,in which A is the
Q117: Given that<br>O(g)+ e<sup>-</sup> → O<sup>-</sup>(g); ΔH =