Given the molecular orbital diagram for dinitrogen (N2) excluding the K shells below,which of the following molecules or ions is expected to be diamagnetic? 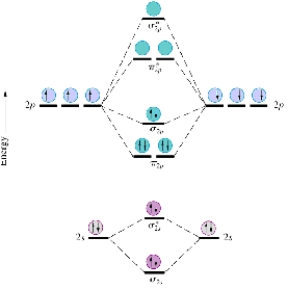
Definitions:
Moving Company
A business that provides services to transport goods and household items from one location to another.
Margin of Error
An indicator of the precision of an estimate in survey data, showing the range within which the true value is expected to lie with a certain probability.
Confidence Interval
A range of values, derived from sample statistics, that is believed to contain the true population parameter with a certain probability.
Confidence Interval
A statistical range, based on sample data, that is likely to contain the true value of an unknown population parameter.
Q1: Consider the following gas-aqueous liquid equilibrium for
Q2: All of the following species are isoelectronic
Q15: Which of the following statements concerning the
Q25: Carbon monoxide is toxic because it can
Q33: Which of the following corresponds to the
Q53: Which of the following compounds would be
Q66: If four orbitals on one atom overlap
Q90: Under conditions of constant pressure,for which of
Q99: A concentrated acetic acid solution has a
Q112: What is the ground-state electron configuration of