Using two or more of the following,
N2(g) + 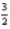
O2(g) → N2O3(s) ; ΔH° = 83.7 kJ
N2(g) + O2(g) → 2NO(g) ; ΔH° = 180.4 kJ 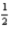
N2(g) + O2(g) → NO2(g) ; ΔH° = 33.2 kJ 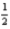
N2(g) + 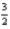
H2(g) → NH3(g) ; ΔH° = −45.9 kJ
Determine ΔH° for the following reaction.
NO(g) + NO2(g) → N2O3(g)
Definitions:
Net Sales
The revenue from goods or services sold after deducting returns, allowances for damaged or missing goods, and any discounts allowed.
Cost of Goods Sold
The specific expenses borne for the production of products a company sells, covering materials and workforce.
Income Tax Expense
The cost associated with taxes on earnings, calculated based on the applicable tax rates and laws.
Comparative Balance Sheet
A financial statement that presents the financial position of a company at two or more different points in time, allowing for comparison and analysis of changes.
Q11: Which of the following is a strong
Q24: Which of the following statements is true
Q40: The angular momentum quantum number is best
Q70: Which molecule or ion has a trigonal
Q75: Which of the following statements about the
Q77: Which of the following statements concerning a
Q86: What is the standard enthalpy change for
Q91: A sample of oxygen is collected over
Q112: Given the following thermochemical data at 25°C
Q130: SO<sub>2</sub> reacts with H<sub>2</sub>S as follows:<br>2H<sub>2</sub>S +