Consider the following gas-aqueous liquid equilibrium for a closed system at a constant temperature.
O2(g) 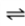 O2(aq)
O2(aq)
What is the effect on the equilibrium composition of the liquid when the partial pressure of O2 gas above the liquid is decreased?
Definitions:
Owner's Equity
The residual interest in the assets of an entity that remains after deducting its liabilities. It represents the ownership interest of the shareholders in a company.
Total Liabilities
The sum of all financial obligations or debts a company owes, including loans, accounts payable, mortgages, and any other monies due to external parties.
Total Assets
The sum of all assets owned by an entity, including both current and noncurrent assets.
Office Equipment
Assets such as computers, desks, chairs, and printers used in an office setting to facilitate operations.
Q11: Which of the following statements is incorrect?<br>A)The
Q11: What is the mole fraction of urea
Q33: What is the concentration of HC<sub>2</sub>O<sub>4</sub><sup>-</sup> in
Q35: When a solid undergoes a phase change
Q51: Which of the following substances has the
Q58: An atom of which of the following
Q59: What is the K<sub>c</sub> equilibrium-constant expression for
Q70: A solution has a pOH of 5.20.What
Q75: Which of the following methods cannot be
Q139: For which of the following equilibria does