Consider the following gas-aqueous liquid equilibrium for a closed system at a constant temperature.
O2(g) 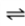 O2(aq)
O2(aq)
What is the effect on the equilibrium composition of the liquid when the partial pressure of O2 gas above the liquid is decreased?
Definitions:
Inventory
Merchandise on hand (not sold) at the end of an accounting period.
Work Around Products
Alternative solutions or products developed to circumvent problems or limitations in existing processes.
Lean Manufacturing
An organized approach for reducing waste in a manufacturing system without compromising on productivity.
Pull Manufacturing
An important lean practice in which products are manufactured only as they are needed by the customer.
Q15: Which of the following statements concerning the
Q23: Which of the following pure substances has
Q24: What is the hydronium-ion concentration of a
Q28: Identify the van der Waals equation.<br>A) <img
Q36: Which of the following Lewis structures best
Q48: Which principle or rule is violated by
Q74: Which of the following best describes carbon
Q116: What type of colloid is formed when
Q121: Which of the following compounds has the
Q121: Which of the following species represents an