The gas-phase decomposition of N2O5 is a first-order process with a rate constant of 1.50 × 10-3 s-1 at 55°C.The decomposition reaction is
N2O5(g) → 2NO2(g) + 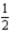
O2(g)
If 8.0 g of N2O5 is placed in vessel 1 and 16.0 g of N2O5 in vessel 2 and the vessels are at the same temperature (55°C) and the same pressure,how much time is required for half of the N2O5 to decompose in each vessel?
Definitions:
George Homans
An American sociologist known for his work in developing social exchange theory, which explains social behavior in terms of the exchange of goods, services, and recognition.
Value
The importance, worth, or usefulness of something, often influenced by social norms and individual perceptions.
Less Valuable
Considered to have lower worth or importance in comparison to something else.
George Homans
An American sociologist known for his work in developing social exchange theory, which posits that the exchange of goods, services, and social values plays a key role in the formation of social relationships.
Q7: Which of the following species cannot act
Q11: A low melting solid readily dissolves in
Q16: What pH should a solution have if
Q36: At 700 K,K<sub>p</sub> for the following equilibrium
Q37: Ammonia is prepared industrially by the reaction<br>N<sub>2</sub>(g)+
Q51: At 400 K,an equilibrium mixture of H<sub>2</sub>,I<sub>2</sub>,and
Q57: A(n)_ is a homogeneous mixture of two
Q63: Which of the following covalent molecules does
Q116: Which of the following equilibria best represents
Q122: For which of the following equilibria does