Given the following equilibrium constants,
Zn(IO3) 2 Ksp = 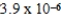
Zn(NH3) 42+ Kf = 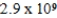
Determine Kc for the dissolution of the sparingly soluble salt Zn(IO3) 2 in aqueous ammonia (shown below) .
Zn(IO3) 2(s) + 4NH3(aq)  Zn(NH3) 42+(aq) + 2IO3-(aq)
Zn(NH3) 42+(aq) + 2IO3-(aq)
Definitions:
Express Authority
Explicit permission given by a principal to an agent to act on their behalf.
Advance Payment
A prepayment made before the actual due date or before receiving the goods or services it is meant for.
Terminated Agency
The end of a legal relationship between a principal and an agent, where the agent can no longer act on behalf of the principal.
Secured Lending
A loan or extension of credit where the borrower pledges an asset as collateral to ensure repayment, reducing the risk for the lender.
Q11: The process of producing a chemical change
Q16: Which one of the following metals reacts
Q17: The concentration of H<sub>3</sub>O<sup>+</sup> in a solution
Q44: What is the value of the reaction
Q60: Which of the following statements is incorrect?<br>A)One
Q64: Which of the following reactions has the
Q71: What is the solubility product expression for
Q79: If a process is both endothermic and
Q101: Equal moles of the indicated acids are
Q116: Which of the following equilibria best represents