Multiple Choice
According to the phase diagram given,which of the following statements is INCORRECT? 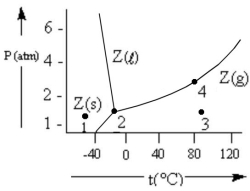
Definitions:
Related Questions
Q6: Of the following elements,which has the lowest
Q20: Using the VSEPR model,the electron-group geometry of
Q21: The specific heat capacity of methane gas
Q29: When 1.50 mol of CH<sub>4</sub>(g)reacts with excess
Q57: Which of the following statements about electron
Q63: An aqueous solution has a normal boiling
Q68: Consider the reaction of 25.0 mL of
Q70: Choose the INCORRECT statement about PCl<sub>5</sub>.<br>A)There are
Q115: For the reaction: H<sub>2</sub>(g)+ I<sub>2</sub>(g)⇌ 2 HI(g),K<sub>c</sub>
Q116: Which compound is likely to be the