Ethanol ( 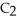
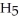 OH) melts at -114 °C.The enthalpy of fusion is 5.02 kJ mol-1.The specific heats of solid and liquid ethanol are 0.97 J g-1 °C-1 and 2.3 J g-1 °C-1,respectively.How much heat (kJ) is needed to convert 25.0 g of solid ethanol at -135 °C to liquid ethanol at -50 °C?
OH) melts at -114 °C.The enthalpy of fusion is 5.02 kJ mol-1.The specific heats of solid and liquid ethanol are 0.97 J g-1 °C-1 and 2.3 J g-1 °C-1,respectively.How much heat (kJ) is needed to convert 25.0 g of solid ethanol at -135 °C to liquid ethanol at -50 °C?
Definitions:
Public Sector
The portion of an economy that is controlled by national, state, or municipal governments.
Empowerment Program
Organizational initiatives designed to increase employees' feelings of self-worth, decision-making power, and effectiveness within the workplace.
Productivity Results
Quantitative outcomes measuring how effectively individuals or organizations convert inputs into outputs, typically in terms of efficiency and effectiveness.
Assembly Workers
Individuals who specialize in putting together components of products in manufacturing plants or factories.
Q9: Choose the INCORRECT statement.<br>A)A certain amount of
Q28: What is the pH of a 0.380
Q29: Which compound would be expected to have
Q52: Choose the INCORRECT statement.<br>A)The core electrons are
Q64: Calculate the pH for an aqueous solution
Q69: Which of the following statements concerning aqueous
Q87: What is the mole fraction of I<sub>2</sub>
Q95: If some NH<sub>4</sub>Cl is added to an
Q99: How many bond pairs ("bp")and how many
Q121: The K<sub>b</sub> value for methylamine is 4.2